This clinical pharmacology summary on sulfonylureas focuses on the pharmacodynamic/pharmacokinetic properties and how to switch/transfer between sulfonylurea agents.
Table of Contents:
3. Sulfonylurea equivalent dose conversion chart
Mechanism of Action
Sulfonylureas are prescribed in the treatment of type 2 diabetes mellitus. Their primary mechanism of action is to stimulate insulin release from the pancreatic beta cells; for this reason, they are only effective when the patient has some residual pancreatic beta cell activity.
The sulfonylureas act by closing the ATP-sensitive potassium (KATP) channels in the cell membrane of the pancreatic beta cells and therefore cause: membrane depolarisation, calcium influx and insulin release. Normally, the KATP channels close when the intracellular levels of glucose increases; firstly, glucose enters the pancreatic beta cells via the glucose transporters (GLUT2), secondly, it is phosphorylated by the enzyme glucokinase and finally, it is metabolised which generates ATP and the subsequent closure of the KATP channels.
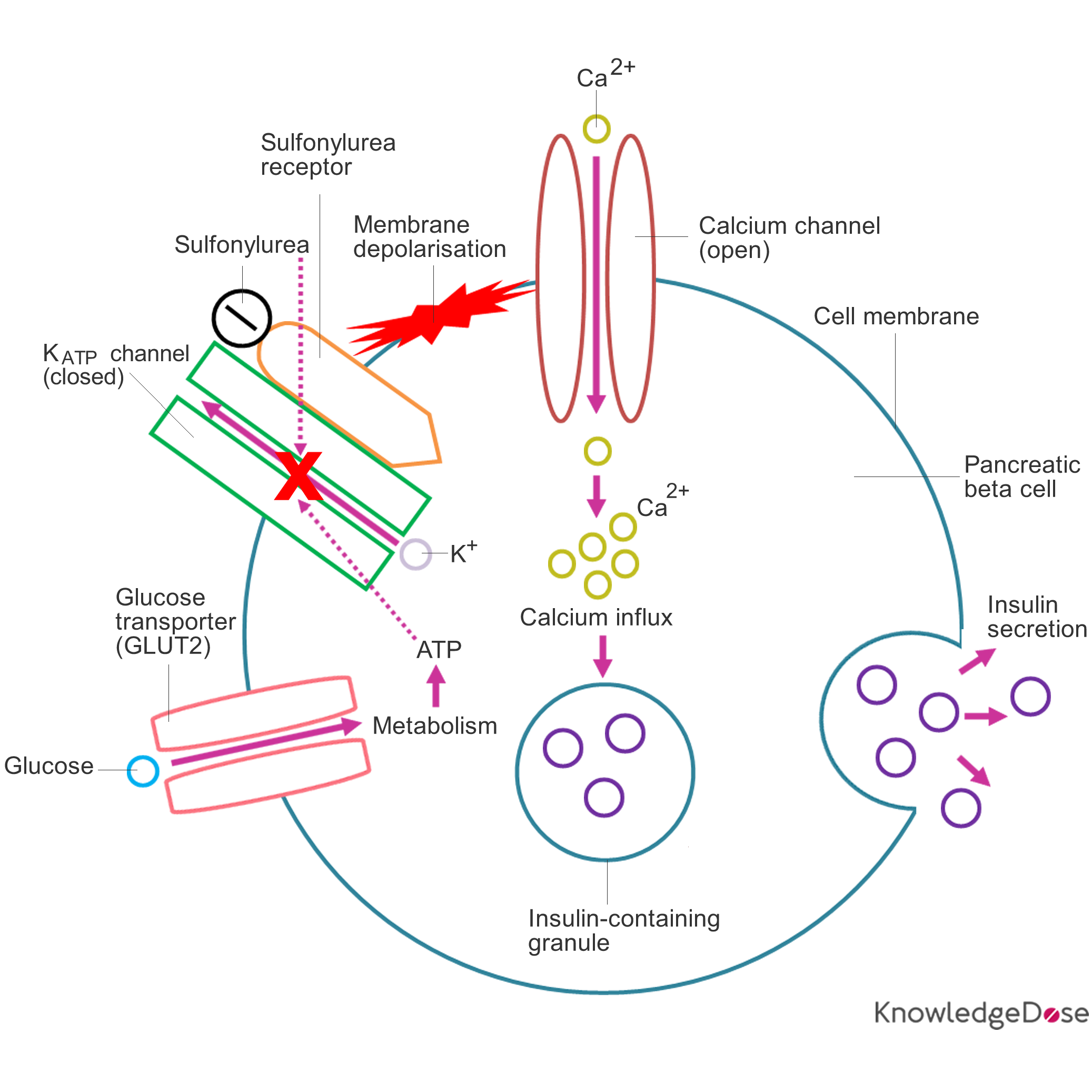
Diagram: Schematic representation of sulfonylureas and their site of action
1) A sulfonylurea binds to the sulfonylurea receptor.
2) The closing of the KATP channels inhibits the efflux of potassium ions through the KATP channels resulting in membrane depolarisation.
3) Membrane depolarisation opens the calcium channels in the cell membrane of the pancreatic beta cell allowing the influx of calcium ions through the calcium channels.
4) The calcium concentration inside the pancreatic beta cell increases, leading to the exocytosis of insulin-containing granules.
List of First- and Second-Generation Sulfonylureas
There are two types of sulfonylureas, the first- and second-generation. The table below lists the first- and second-generation sulfonylureas available in the UK for the treatment of type 2 diabetes mellitus.
Table: Sulfonylurea agents and their pharmacokinetic properties
|
Sulfonylurea |
Generation |
Pharmacokinetic properties |
|---|---|---|
|
Tolbutamide |
First |
Duration of action: 6-12 hours (short-acting sulfonylurea), Half-life (t1/2): 4-8 hours, Metabolism: Metabolised in the liver by cytochrome P450 isoenzyme (CYP2C9), Highly protein bound: Yes |
|
Glibenclamide |
Second |
Duration of action: Up to 24 hours (long-acting sulfonylurea), Half-life (t1/2): 10 hours, Metabolism: Metabolised mainly in the liver, Highly protein bound: Yes |
|
Glipizide |
Second |
Duration of action: Up to 24 hours (short-acting sulfonylurea), Half-life (t1/2): 2-4 hours, Metabolism: Metabolised mainly in the liver, Highly protein bound: Yes |
|
Gliclazide |
Second |
Duration of action: 10-24 hours (short-acting sulfonylurea), Half-life (t1/2): 10-12 hours (standard release) and 12-20 hours (MR), Metabolism: Metabolised in the liver, Highly protein bound: Yes |
|
Glimepiride |
Second |
Duration of action: 12-24 hours (short-acting sulfonylurea), Half-life (t1/2): 5-8 hours, Metabolism: Metabolised in the liver, Highly protein bound: Yes |
Source: References 1-6
The main side effects of sulfonylureas are:
- Hypoglycaemia, this is more common with long-acting sulfonylureas. Other risk factors for developing hypoglycaemia include: increasing age, skipping meals, weight loss and impaired renal or hepatic function.
- Gastrointestinal disturbances e.g. nausea, vomiting, diarrhoea
- Weight gain
Patients prescribed sulfonylureas should be advised about the possible risk of hypoglycaemia. If sulfonylureas are prescribed in the elderly, a short-acting sulfonylurea is recommended due to their short half-life; tolbutamide can safely be prescribed in elderly patients. Conversely, glibenclamide should be avoided in the elderly as it is long-acting and has a greater risk of hypoglycaemia.
Sulfonylurea Equivalent Doses / Switching
Switching between gliclazide MR and gliclazide standard release
To switch/transfer from gliclazide standard release to gliclazide MR (modified release):
- Gliclazide 80mg standard release is approximately equivalent to gliclazide 30mg MR
- The manufacturers recommend that blood glucose is monitored
Equivalent dose data derived from: Servier Laboratories Limited. Diamicron MR 30 mg – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/1321/smpc [Accessed on 04/10/19].
Read the full prescribing advice on how to switch from other oral hypoglycaemic agents to gliclazide MR 30mg.
Sulfonylurea equivalent dose chart
To switch/transfer from one sulfonylurea to another, the approximate equivalent doses are shown in the dose chart below:
|
Sulfonylurea equivalent doses |
|---|
|
Glibenclamide 5mg is approximately equivalent to: |
|
1000mg tolbutamide |
|
250mg chlorpropamide* |
|
5mg glipizide |
*Chlorpropamide has been discontinued in the UK
Approximate equivalent doses presented in the table derived from: Wockhardt UK Ltd. Glibenclamide 2.5mg Tablets – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/6839/smpc [Accessed on 04/10/19].
Read the full prescribing advice on switching over from other sulfonylureas, metformin and insulin.
References:
1. Accord Healthcare Limited. Glimepiride 1 mg Tablets – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/6053/smpc [Accessed on 04/10/19].
2. Accord-UK Ltd. Tolbutamide Tablets BP 500mg – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/5968 [Accessed on 04/10/19].
3. Mylan. Glipizide 5 mg Tablets – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/8543/smpc [Accessed on 04/10/19].
4. National Center for Biotechnology Information. PubChem Database. Glyburide, CID=3488. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Glyburide [Accessed on 04/10/19].
5. Servier Laboratories Limited. Diamicron 80mg Tablets – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/1150/smpc [Accessed on 04/10/19].
6. Servier Laboratories Limited. Diamicron MR 30 mg – Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/1321/smpc [Accessed on 04/10/19].